Glycochemistry Structures
Foliensatz 2 SoSe 2020
Foliensatz 2 SoSe 2020
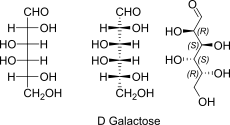
D-Galactose openchain to alpha/beta D Galactopyranose (anomeric reference stereocenter configuration?)
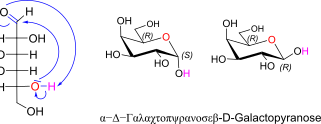
lol kein plan was da mit der schriftart passiert ist. btw: C4 ist (S) confi!
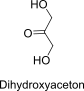
Dihydroxyaceton
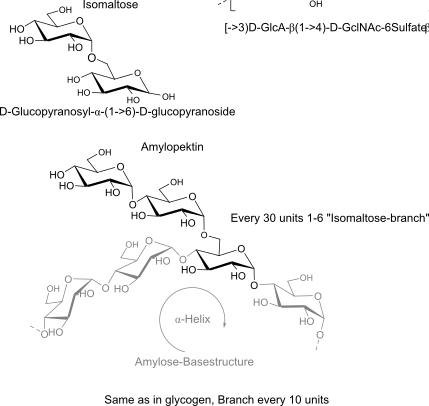
Isomaltose:
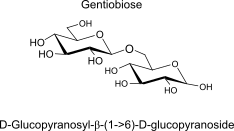
units, binding, polymer (name, binding, H-bonds, repeats, 3D-structure -> properties)
Komisches kopier artefakt oben rechts ignorieren. danke :)
D-Galactose open chain Fischer + absolute stereo
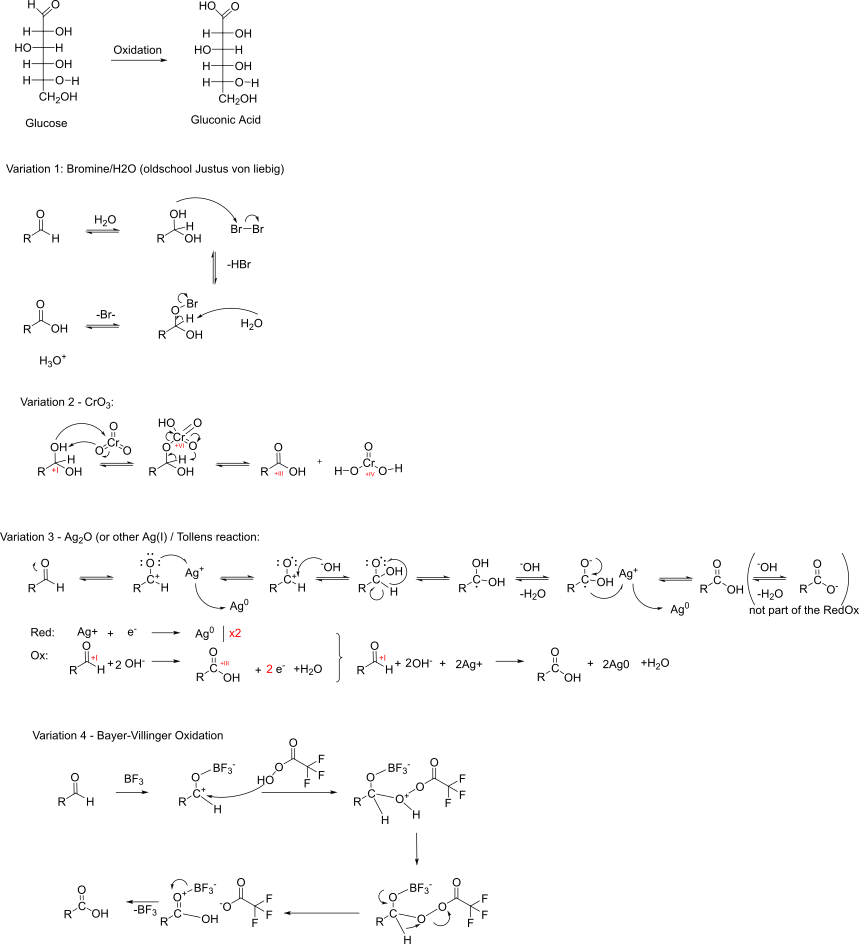
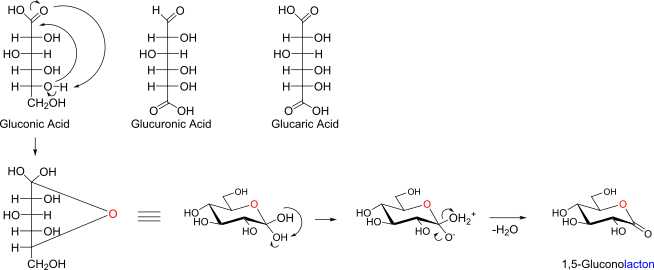
Synthesis of Gluconic Acid
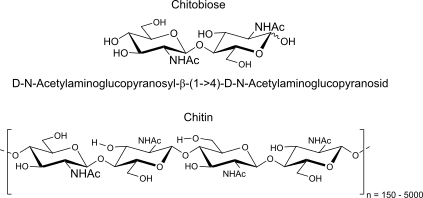
Chitobiose:
units, binding, polymer (name, binding, H-bonds, repeats)
reducing
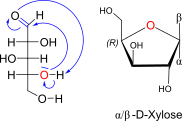
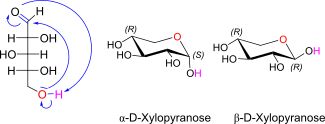
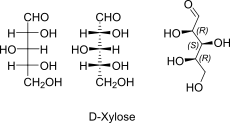
D-Xylose openchain to alpha/beta D Xyloopyranose (anomereic reference stereocenter configuration?)
(= Glucose without C5-CH2OH)
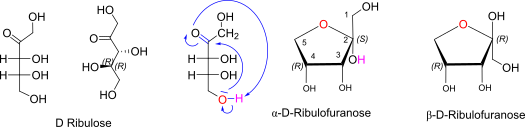
D-ribulose openchain to alpha/beta D Ribulofuranose (anomeric reference stereocenter configuration?)
Deoxyribofuranose

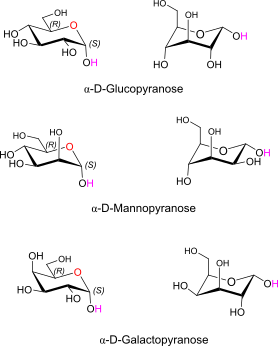
Glc, Man, Gal in D alpha pyranose form 4C1 and 1C4 conformation (Ring flip)
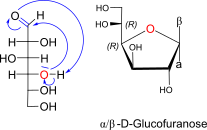
D-Glucose openchain to alpha / beta D Glucofuranose
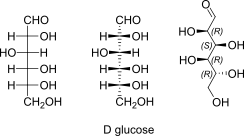
D-Glucose open chain Fischer + absolute stereo
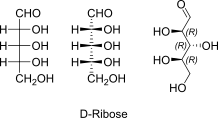
D-Ribose open chain Fischer + absolute stereo
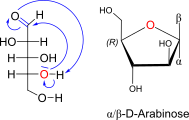
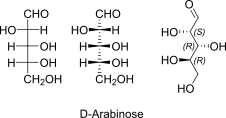
D-Arabinose openchain to alpha/beta D Arabinopyranose (anomereic reference stereocenter configuration?)
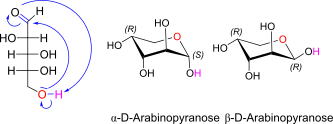
Note: L-arabinopyranose is thermodyn favored (= C4 OH axial, C2/C3-OH equatorial!)
Or D-4C1 form
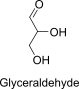
Glyceraldehyde
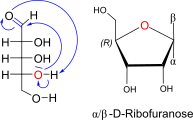
D-Ribose openchain to alpha / beta D Ribofuranose
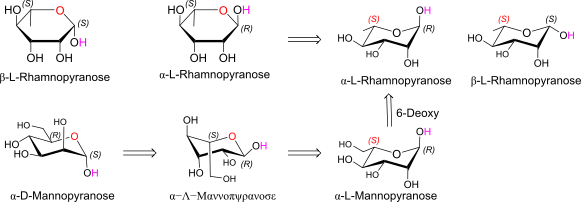
alpha / beta L rhamnose (Harworth and Chair)
Relation to Mannose?
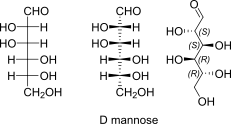
D-mannose open chain Fischer + absolute stereo
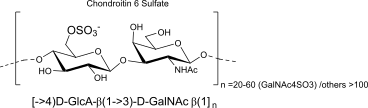
Chondroitin 6 Sulfate
units, binding, polymer (name, binding, repeats)
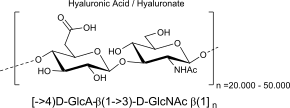
Hyaluronic Acid:
units, binding, polymer (name, binding)
Glucosamine and N-Acetyl glucosamine (Abbrev.?)

Gentiobiose
D-Arabinose openchain to alpha / beta D Arabinofuranose
Carbohydrate chain elongation of D-Arabinose
- Synt / Mechanism
- Synt-Name
-Stereo outcome - Hexose names
-reverse reaction name
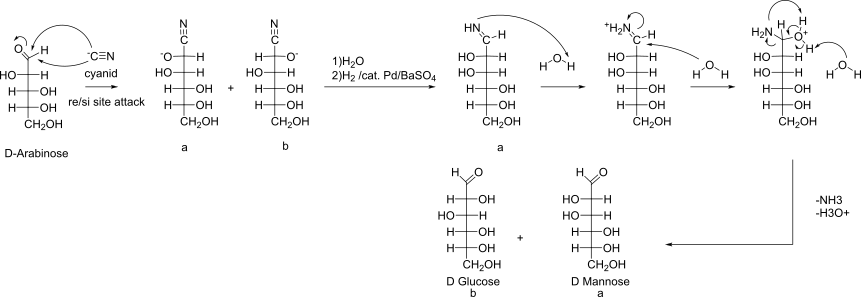 Killiani-Fischer Chain elongation - Optimized Variation by Kuhn
Killiani-Fischer Chain elongation - Optimized Variation by Kuhn
C2-Epimers as product
Reverse: Ruff-Degradation -> Oxidation of Aldehyde with Water/Br2, then Decarboxylation by Fe2+/H2O2 (Radikal-mechanism)
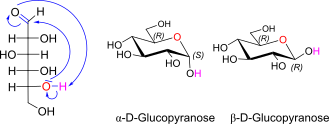
D-Glucose openchain to alpha/beta D Glucopyranose (anomeric reference stereocenter configuration?)
Maltose:
units, binding, polymer (name, binding, H-bonds, repeats, 3D-structure -> properties)
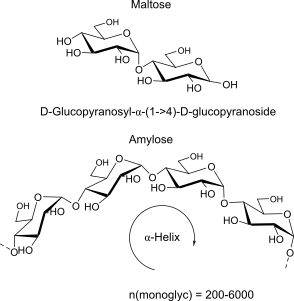
alpha helix structure = hydrophilic outside, hydrophobic inside => Cyclodextrines!
Cellobiose:
units, binding, reducing/nonreducing?, Polymer (name, binding, H-bonds, repeats)
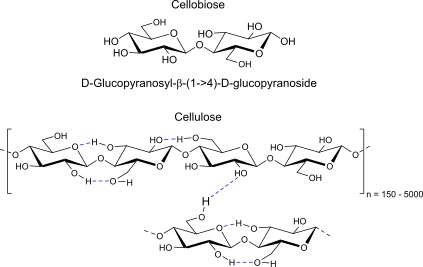
reducing
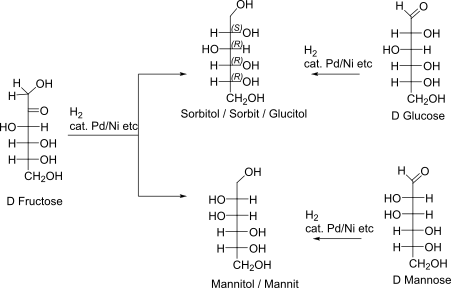
Sorbitol and Mannit
Synthesis from Parent sugars
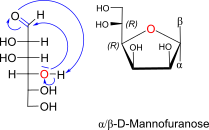
D-Mannose openchain to alpha / beta D Mannofuranose
D-Galactose openchain to alpha / beta D Galactofuranose

L-Fucose and relation to Galactose

D-Xylose open chain Fischer + absolute stereo
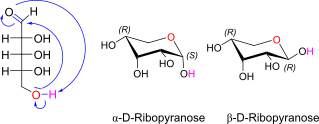
D-Ribose openchain to alpha/beta D Ribopyranose (anomereic reference stereocenter configuration?)
Succrose
red / non red?

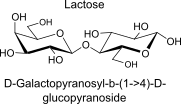
Lactose
non reducing or reducing?
reducing
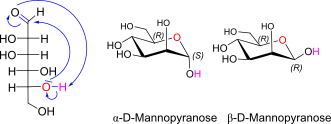
D-Mannose openchain to alpha/beta D Mannose (anomeric reference stereocenter configuration?)
On/Uron/Ar-Acids of glucose and cyclisation of Gluconic acid (name of product)
D-Fructose openchain to alpha/beta D Fructopyranose (anomeric reference stereocenter configuration?)
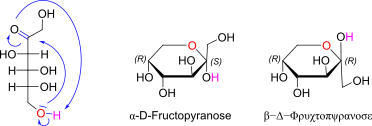
nices ß anomer, immerhin nicht comic sans
D-Arabinose open chain Fischer + absolute stereo
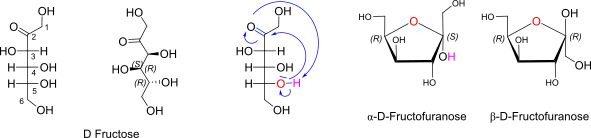
D-Fructose openchain to alpha/beta D Fructofuranose (anomeric reference stereocenter configuration?)
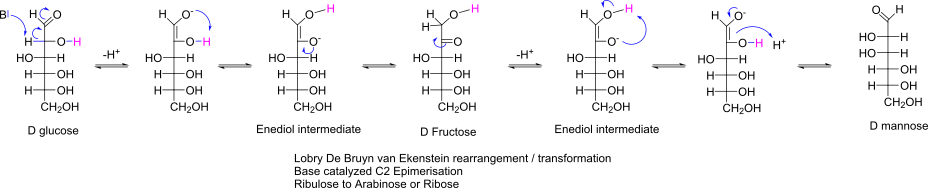
Tautomery of Glucose with Mannose (mechanism)
Name of the reaction
Names of the Tautomers
Name of the intermediate species
Stereochemical outcome
Which Pentoses do you get from Ribulose in this reaction?
Trehalose / Mykose
red / non-red?

non-reducing
D-Xylose openchain to alpha / beta D Xylofuranose